In recent years, the aging population has led to a significant increase in chronic renal diseases, which are prevalent among elderly individuals. Key diagnostic indicators for chronic renal disease include blood creatinine, blood urea nitrogen, urine protein, and blood oxygen saturation. Among these, blood oxygen saturation is a critical measure of oxygen content in the blood. Patients with kidney disease may experience complications such as anemia and cardiovascular issues due to renal damage, affecting blood oxygen levels and thus blood oxygen saturation. Monitoring blood oxygen saturation aids in clinical assessment and timely intervention. With advancements in robotics, control technology, and artificial intelligence, medical robots offer efficient and intelligent assistance to healthcare professionals, improving the quality and efficiency of medical services. These medical robots are strategically important for the future of healthcare. However, when medical robots operate, they must interact safely with patients, requiring compliant control capabilities. Medical robots are complex multi-body systems, posing challenges for designing high-safety, high-precision motion controllers.
For robot motion control, common techniques include fuzzy control, adaptive control, model predictive control, and sliding mode control (SMC). However, these approaches often neglect the interaction between the robot end-effector and the target, failing to ensure controllable contact forces. Impedance control allows medical robots to adjust their impedance (resistance and motion inertia) based on environmental and task variations, achieving safer and more efficient control. For instance, Fayazi et al. used fractional-order SMC to design an impedance controller for a single-degree-of-freedom flexible robot, enabling effective end-effector trajectory tracking. Rayguru et al. combined time-delay estimation with SMC to develop impedance control for a six-degree-of-freedom robot, enhancing adaptability to contact environments. While SMC is simple and suitable for designing robot impedance controllers, conventional SMC does not guarantee finite-time convergence and suffers from chattering. Fast terminal SMC (FTSMC) ensures rapid finite-time convergence and has been successfully applied to robot trajectory tracking. To address singularity issues caused by negative fractional power terms in FTSMC, Jouila et al. proposed non-singular FTSMC (NFTSMC), which offers fast convergence, avoidance of singularities, and reduced chattering. Since human tissue is not rigid, medical robots require adaptive capabilities when the detection end makes contact. Additionally, to ensure timely blood oxygen saturation detection, controllers must exhibit fast response and precision.
Based on this analysis, we propose an adaptive non-singular fast terminal sliding mode (ANFTSMC) impedance control scheme to meet the control requirements of medical robots for autonomous chronic renal disease detection. By designing a system prototype, explaining the working principle, and establishing a dynamic model, we clarify the input-output relationships. Then, following a model-free approach, we design a trajectory tracking controller to achieve compliant control of the medical robot. Finally, simulation examples validate the effectiveness and superiority of the proposed controller.
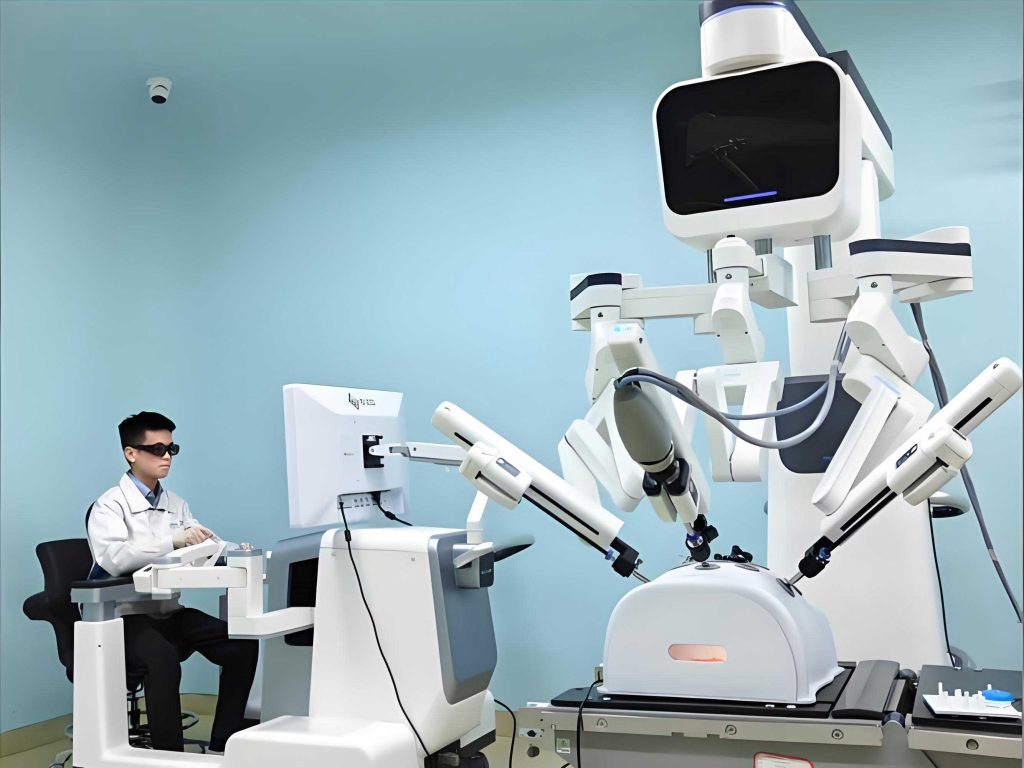
In the context of “Internet + healthcare,” we aim to develop an unmanned intelligent medical robot detection station for auxiliary diagnosis of chronic renal disease in elderly populations in remote areas such as rural and urban regions. Using blood oxygen saturation detection as an example, the system workflow is as follows: First, users enter the detection station via identification, lie on the detection table, and press a start button. The medical robot automatically positions the end detection end near the target area (e.g., fingertip, earlobe, nose, toe) based on camera feedback of human features to acquire blood oxygen saturation information. Then, via Bluetooth modules, the blood oxygen saturation data from multiple body parts are uploaded to the cloud. Next, hospital terminal analysis centers download the detection information from the cloud and automatically generate detection reports. Staff can preliminarily diagnose whether users have complications related to kidney disease based on the reports. If abnormalities are detected, staff notify users for further hospital re-examination. This system alleviates hospital patient flow, reduces labor costs, and improves diagnostic efficiency.
The core component of this system is the medical robot. To ensure the end detection end fully contacts the target area, we select four degrees of freedom. The arm is lightweight to reduce inertia. The medical robot has a serial topology consisting of a base, four joint axes, upper arm, forearm, end-effector, and end detection end. This medical robot features fast response, large workspace, and high flexibility. The Denavit-Hartenberg (DH) parameters are summarized in Table 1.
| Joint i | Link Length a_i (mm) | Link Twist α_i (°) | Link Offset d_i (mm) | Joint Angle q_i (°) |
|---|---|---|---|---|
| 1 | 0 | 90 | -104 | q1 |
| 2 | 150 | 0 | 0 | q2 |
| 3 | 120 | 0 | 0 | q3 |
| 4 | 50 | 0 | 0 | q4 |
The dynamic model of the four-degree-of-freedom medical robot for automatic blood oxygen saturation detection is derived using the Newton-Euler equation:
$$ M(q) \ddot{q} + C(q, \dot{q}) \dot{q} + G(q) = \tau + \tau_d $$
where \( q, \dot{q}, \ddot{q} \in \mathbb{R}^4 \) represent joint angular displacement, velocity, and acceleration, respectively; \( \tau, \tau_d \in \mathbb{R}^4 \) are joint control torque and disturbance torque; \( M(q) \in \mathbb{R}^{4 \times 4} \) is a symmetric positive definite matrix; \( C(q, \dot{q}) \in \mathbb{R}^{4 \times 4} \) denotes Coriolis and centrifugal terms; and \( G(q) \in \mathbb{R}^4 \) is the gravity term.
Assuming \( M_0(q) \), \( C_0(q, \dot{q}) \), and \( G_0(q) \) are nominal terms, and \( \Delta M(q) \), \( \Delta C(q, \dot{q}) \), and \( \Delta G(q) \) are unmodeled dynamics, Equation (1) can be rewritten as:
$$ M_0(q) \ddot{q} + C_0(q, \dot{q}) \dot{q} + G_0(q) = \tau + F_d $$
where \( F_d = \tau_d – \Delta M(q) \ddot{q} – \Delta C(q, \dot{q}) \dot{q} – \Delta G(q) \) represents the lumped disturbance term encompassing unmodeled dynamics and external disturbances.
Further, mapping the dynamic model from joint space to workspace yields:
$$ M_P(q) \ddot{P} + C_P(q, \dot{q}) \dot{P} + G_P(q) = F_d + F_P $$
where \( M_P(q) = J^{-T} M(q) J^{-1} \), \( G_P(q) = J^{-T} G(q) \), \( F_P = J^{-T} \tau \), \( C_P(q, \dot{q}) = J^{-T} (C(q, \dot{q}) – M(q) J^{-1} \dot{J}) J^{-1} \), and \( J \) is the Jacobian matrix. \( P \in \mathbb{R}^{6 \times 1} \) denotes the position and orientation of the end detection end in workspace.
When the medical robot’s end detector contacts human tissue, the relationship between end contact force and position is modeled as a spring-mass-damper system. By adjusting damping coefficient \( m \), stiffness coefficient \( b \), and spring coefficient \( k \), compliant control of the medical robot is achieved. Defining \( F_e \) as the contact force:
$$ m (\ddot{P}_c – \ddot{P}) + b (\dot{P}_c – \dot{P}) + k (P_c – P) = F_e $$
where \( P_c, \dot{P}_c, \ddot{P}_c \) are reference trajectory position, velocity, and acceleration, and \( P, \dot{P}, \ddot{P} \) are actual values.
Combining Equations (1) to (4), the final dynamic model of the medical robot is:
$$ M_P(q) \ddot{P} + C_P(q, \dot{q}) \dot{P} + G_P(q) – F_d = F_P – F_e $$
To design the impedance sliding mode controller, define position tracking error as \( e = P – P_d \), with first and second derivatives \( \dot{e} = \dot{P} – \dot{P}_d \) and \( \ddot{e} = \ddot{P} – \ddot{P}_d \). From Equation (5), we have:
$$ \ddot{e} = M_P^{-1}(q) [F_P + F_d – C_P(q, \dot{q}) \dot{q} – G_P(q)] – \ddot{q}_d $$
Differentiating further:
$$ \dddot{e} = \dot{M}_P^{-1}(q) [F_P – C_P(q, \dot{q}) \dot{q} + G_P(q)] + M_P^{-1}(q) \left[ \dot{F}_P – \frac{d}{dt}(C_P(q, \dot{q}) \dot{q} + G_P(q)) \right] – \dddot{q}_d + \dot{F}_d $$
Assuming the lumped disturbance \( F_d \) is bounded:
$$ \| F_d \| \leq v_0 + v_1 \| q \| + v_2 \| \dot{q} \|^2 $$
where \( v_0, v_1, v_2 \) are positive constants.
We design a linear sliding surface:
$$ \sigma = \dot{e} + \epsilon e $$
where \( \sigma \in \mathbb{R}^4 \) is the linear sliding surface, and \( \epsilon \in \mathbb{R}^{4 \times 4} \) is a positive definite diagonal matrix.
The ANFTSMC sliding surface is defined as:
$$ s = \dot{\sigma} + k_1 \text{sig}^\alpha(\sigma) + k_2 \text{sig}^\beta(\sigma) $$
where \( s \in \mathbb{R}^4 \) is the ANFTSMC sliding surface, \( k_1 > 0 \), \( k_2 > 0 \), \( 1 < \beta < 2 \), \( \alpha > \beta \), and \( \text{sig}^\alpha(\sigma) = |\sigma|^\alpha \text{sign}(\sigma) \), with \( \text{sign}(\cdot) \) as the sign function.
Following literature, the derivative of \( s \) is:
$$ \dot{s} = \ddot{\sigma} + \alpha k_1 |\sigma|^{\alpha-1} \dot{\sigma} + \beta k_2 |\sigma|^{\beta-1} \dot{\sigma} $$
Differentiating Equation (9) and combining with Equations (5) and (11), the derivative of equivalent control torque \( f_{eq} \) is:
$$ \dot{f}_{eq} = \frac{d}{dt} \left( C_P(q, \dot{q}) \dot{q} + G_P(q) \right) + M_P(q) \dddot{q}_d – \epsilon M_P(q) \ddot{e} – M_P(q) \dot{M}_P^{-1}(q) \left[ F_P – C_P(q, \dot{q}) \dot{q} + G_P(q) \right] – M_P(q) \left( \alpha k_1 |\sigma|^{\alpha-1} \dot{\sigma} + \beta k_2 |\sigma|^{\beta-1} \dot{\sigma} \right) $$
Introducing a fast convergence control torque \( f_{co} \), its derivative is:
$$ \dot{f}_{co} = -M_P(q) \left( \hat{v}_0 + \hat{v}_1 \| q \| + \hat{v}_2 \| \dot{q} \|^2 \right) \text{sign}(s) $$
where \( \hat{v}_0, \hat{v}_1, \hat{v}_2 \) are estimates of \( v_0, v_1, v_2 \), updated by the adaptive mechanism:
$$ \begin{aligned}
\dot{\hat{v}}_0 &= \| s \| \\
\dot{\hat{v}}_1 &= \| s \| \| q \| \\
\dot{\hat{v}}_2 &= \| s \| \| \dot{q} \|^2
\end{aligned} $$
Combining Equations (12) and (13), the control torque is:
$$ F_P = \dot{f}_{eq} + \dot{f}_{co} = \int (\dot{f}_{eq} + \dot{f}_{co}) \, dt $$
Considering impedance control torque, the overall control torque for the medical robot is:
$$ F_c = F_P – F_e $$
For stability analysis, consider the Lyapunov function:
$$ V = \frac{1}{2} s^2 + \frac{1}{2} \left[ (\hat{v}_0 – v_0)^2 + (\hat{v}_1 – v_1)^2 + (\hat{v}_2 – v_2)^2 \right] $$
Differentiating:
$$ \dot{V} = s \dot{s} + (\hat{v}_0 – v_0) \dot{\hat{v}}_0 + (\hat{v}_1 – v_1) \dot{\hat{v}}_1 + (\hat{v}_2 – v_2) \dot{\hat{v}}_2 $$
Substituting Equations (7) and (11):
$$ \dot{V} \leq \| s \| \left( F_d – (\hat{v}_0 + \hat{v}_1 \| q \| + \hat{v}_2 \| \dot{q} \|^2) \right) + (\hat{v}_0 – v_0) \| s \| + (\hat{v}_1 – v_1) \| s \| + (\hat{v}_2 – v_2) \| s \| = \| s \| (F_d – v_0 – v_1 \| q \| – v_2 \| \dot{q} \|^2) $$
Let \( \zeta = F_d – v_0 – v_1 \| q \| – v_2 \| \dot{q} \|^2 \). From Equation (8), \( \zeta \leq 0 \). Thus, \( \dot{V} \leq 0 \), proving boundedness of sliding surface \( s \).
Consider another Lyapunov function:
$$ V_1 = \frac{1}{2} s s^T $$
Differentiating:
$$ \dot{V}_1 = \zeta \| s \| + (\hat{v}_0 – v_0) \dot{\hat{v}}_0 + (\hat{v}_1 – v_1) \dot{\hat{v}}_1 + (\hat{v}_2 – v_2) \dot{\hat{v}}_2 $$
Assuming finite time \( T_1 \) such that \( \hat{v}_i \geq v_i \) for \( t \geq T_1 \), or initially \( \hat{v}_i \geq v_i \), we obtain \( \dot{V}_1 \leq \zeta \| s \| \). Therefore:
$$ \dot{V}_1 \leq – \sqrt{2} |\zeta| V_1^{1/2}, \quad t \geq T $$
where \( T \) is a bounded time constant. According to Lyapunov stability theory, system states converge asymptotically to \( s = 0 \), and position tracking error \( e \) converges to zero along the linear sliding surface \( \sigma \).
To validate the proposed controller, we compare it with PID and conventional SMC through simulations. The parameters for all controllers are listed in Table 2. Simulations are conducted on an Inter Core i7-4720 PC using MATLAB/Simulink 2022a. The medical robot model is imported into Simscape from Solidworks, with structural parameters automatically extracted. The initial state of the end detection end is \( P_1 = (120 \, \text{mm}, 0 \, \text{mm}, -304 \, \text{mm}) \), \( \Theta_1 = (-90^\circ, 0^\circ, 90^\circ) \), and target state is \( P_2 = (109 \, \text{mm}, 130 \, \text{mm}, -343 \, \text{mm}) \), \( \Theta_2 = (-100^\circ, 50^\circ, 90^\circ) \). Contact force between the medical robot end detector and human tissue must not exceed 0.1 N, with damping coefficient \( m = 10 \), stiffness coefficient \( b = 0.1 \), and spring coefficient \( k = 0.01 \). Gaussian noise with mean 0 and variance 0.01 is added to the dynamic model to simulate unmodeled dynamics, and white noise with mean 0 and variance 0.1 is added to control torque to simulate disturbances. Simulation time is 8 s with a sampling frequency of 1000 Hz.
| Controller | Parameters |
|---|---|
| PID | \( K_p = \text{diag}(100, 100, 100, 100) \), \( K_I = \text{diag}(10, 10, 10, 10) \), \( K_D = \text{diag}(80, 80, 80, 80) \) |
| SMC | \( c = \text{diag}(8, 8, 8, 8) \), \( k = 5 \), \( \epsilon = 0.5 \) |
| ANFTSMC | \( k_1 = k_2 = \text{diag}(1, 1, 1, 1) \), \( \alpha = 2 \), \( \beta = 1.5 \), \( \epsilon = \text{diag}(10, 10, 10, 10) \) |
Using inverse kinematics, joint angles for initial and target states are computed, and cubic polynomials generate joint angle trajectories. Forward kinematics yields end-effector positions, visualized via robotics toolbox. The end-effector trajectory in workspace is smooth and continuous, facilitating controller tracking.
To clearly present controller performance, medical robot outputs are mapped from workspace to joint space. The response curves for each joint under PID, SMC, and ANFTSMC show that all three controllers suppress lumped disturbances effectively, enabling joint tracking of reference trajectories. Error performance metrics—maximum error (ME), mean error (MeanE), and root mean square error (RMSE)—are used for quantitative comparison, as summarized in Table 3.
| Controller | ME (°) | MeanE (°) | RMSE (°) |
|---|---|---|---|
| PID | 1.82 | 0.60 | 0.13 |
| SMC | 1.13 | 0.51 | 0.11 |
| ANFTSMC | 0.64 | 0.25 | 0.06 |
ANFTSMC exhibits superior error performance compared to PID and SMC. Based on ME, ANFTSMC has smaller overshoot and better transient performance. Based on MeanE and RMSE, ANFTSMC offers higher tracking accuracy and disturbance rejection, indicating better steady-state performance. For instance, RMSE for ANFTSMC is 53.8% lower than PID and 46% lower than SMC. This improvement stems from the non-singular fast terminal sliding surface in ANFTSMC, which ensures faster convergence than SMC and PID, while the adaptive strategy enhances robustness to lumped disturbances.
Torque response curves for the medical robot under the three controllers show that control torques remain within -0.1 N to 0.1 N, ensuring stable operation. Compared to PID and SMC, ANFTSMC generates control torques with smaller amplitude and smoother curves, indicating reduced chattering. The integral form of ANFTSMC avoids chattering without compromising tracking performance.
During simulation time 6 s to 8 s, the medical robot end detector contacts human tissue for blood oxygen saturation measurement. Contact force responses under all controllers converge to desired values, with ANFTSMC showing the fastest response—settling time approximately 0.5 s, which is 62.5% of SMC and 41.67% of PID. This demonstrates ANFTSMC’s superior control performance. Additionally, ANFTSMC steady-state error stabilizes at 0.002 N, achieving effective force control in the medical robot workspace.
Visualization of the medical robot motion in Simscape shows clear joint angle movements, providing a reference for prototype testing of the medical robot.
In conclusion, we propose an ANFTSMC-based impedance controller for medical robots in contact with human tissue for automatic chronic renal disease detection. ANFTSMC accelerates convergence via a non-singular terminal sliding surface, while integral control ensures continuity and eliminates chattering without affecting tracking performance. Key findings are: (1) ANFTSMC outperforms SMC and PID in transient and steady-state performance, offering better compliant control for medical robots. (2) ANFTSMC ensures finite-time convergence of system states and generates smooth, continuous control torques. (3) The designed medical robot system has engineering value, providing new insights for automatic blood oxygen saturation detection. Future work will involve developing a prototype medical robot for chronic renal disease detection and testing the system in clinical environments to validate effectiveness and feasibility. The integration of advanced control strategies like ANFTSMC is crucial for enhancing the safety and efficiency of medical robots in healthcare applications.
