The silent killer, lung cancer, maintains its grim reign as the world’s deadliest malignancy. Early detection is paramount – survival rates plummet from over 90% in the earliest stages to less than 20% once advanced. Yet, a mere 15% of cases are caught early. Traditional diagnostic tools, like X-rays and CT scans, often fall short in sensitivity, specificity, or safety, while percutaneous biopsies carry significant complication risks. The intricate, tree-like labyrinth of the human bronchial system, with its countless narrow, twisting branches, has long posed an almost insurmountable challenge for precise access to elusive peripheral lung nodules. Enter the transformative era of the bronchoscopic medical robot.
These sophisticated systems represent a quantum leap in medical technology, merging robotics, advanced imaging, and artificial intelligence to navigate the deepest recesses of the lungs with unprecedented precision. Unlike conventional bronchoscopes reliant solely on a physician’s dexterity and interpretation of often ambiguous images, these medical robots integrate robotic arms equipped with high-definition cameras and sensors. This synergy grants physicians enhanced visualization, superior control, and the ability to perform complex biopsies and therapeutic interventions deep within the lung periphery through minimally invasive natural orifices. The core promise is clear: significantly higher diagnostic yields for small, hard-to-reach nodules, reduced procedural risks for patients, shorter learning curves for physicians, and ultimately, more lives saved through earlier intervention.
Bridging the Clinical Gap: From Vision to Reality
The evolution of bronchoscopy mirrors the advancement of the medical robot. Traditional bronchoscopy, while valuable, is limited by operator skill, the difficulty of reaching peripheral lesions, and the challenge of maintaining precise positioning within the complex bronchial tree. Navigation-guided bronchoscopy (e.g., Virtual Bronchoscopic Navigation – VBN, Electromagnetic Navigation Bronchoscopy – ENB) marked a significant step forward, using pre-operative CT scans to create roadmaps and guide instruments electromagnetically or virtually. However, these systems still rely heavily on manual manipulation and can be susceptible to registration errors or “drift” during the procedure.
The integration of robotics is the game-changer. Pioneering platforms like Intuitive Surgical’s Ion and Auris Health’s (now Johnson & Johnson) Monarch medical robots have received FDA and NMPA clearances. These systems go beyond simple guidance; they actively drive the bronchoscope. Monarch employs articulated “snake-like” mechanics for fine control, while Ion utilizes shape-sensing technology via fiber optic sensors embedded within its ultra-thin, flexible scope. Clinical studies indicate these robotic systems outperform traditional and navigation-guided methods in reaching peripheral targets, obtaining adequate biopsy samples, and maintaining diagnostic accuracy, all while potentially lowering complication rates. The medical robot is transitioning from a novel concept to a tangible tool in leading thoracic centers, offering a level of precision and reach previously deemed impossible.
Engineering the Future: Key Technological Pillars
The power of the bronchoscopic medical robot rests on breakthroughs across several critical technological domains:
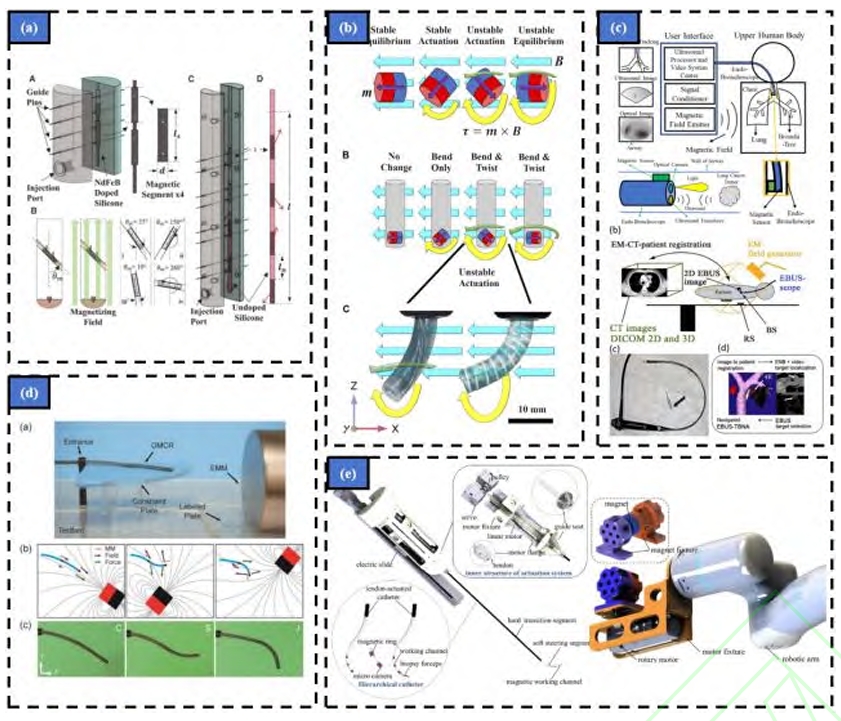
- Flexible Robot Design for Narrow Cavities: The core challenge is creating an instrument slender enough (often sub-4mm diameter) to traverse the smallest bronchioles, yet robust and dexterous enough to be steered accurately and carry working channels for biopsy tools. Research is exploding in this area:
- Motor-Driven Adaptations: Many early platforms retrofit existing bronchoscopes with motorized controls at the proximal end, enabling remote manipulation and providing a testbed for navigation algorithms. This approach leverages existing technology but may lack the ultimate miniaturization needed for the deepest reaches.
- Flexible Continuum Robots: Inspired by structures like elephant trunks or octopus arms, these robots lack rigid joints. Instead, they bend continuously using principles like tendon-driven actuation (pulling cables to induce curvature) or concentric tube designs (interacting pre-curved tubes). Teams globally (e.g., BIT’s Duan Xingguang, CASIA’s Bian Guibin, Vanderbilt’s Webster) are innovating with Ni-Ti alloy tubes, laser-cut patterns, and novel joint designs to maximize bending angles, stiffness control, and miniaturization while incorporating essential tool channels. The medical robot‘s physical form factor is constantly evolving towards greater flexibility and smaller footprints.
- Magnetically Driven Continuum Robots: This cutting-edge approach uses external magnetic fields to steer tiny, untethered or semi-tethered soft robotic probes made from magnetically responsive materials. Researchers (e.g., Kim, Lloyd, Lin, Pittiglio) are developing sub-millimeter robots with self-lubricating coatings, helical reinforcements for stability, and even “opposite-magnetized” designs for enhanced dexterity within confined spaces. The potential for ultra-deep lung access with minimal tissue interaction makes this a highly promising frontier for the next generation of medical robots.
- Dynamic Navigation in Complex Airways: Knowing where the robot is within the constantly shifting bronchial tree during respiration is paramount. Navigation systems combine pre-operative planning with real-time intra-operative tracking:
- Path Planning: The process starts with high-resolution CT scans used to segment and reconstruct a detailed 3D model of the patient’s unique airway tree (“bronchial tree”). Sophisticated algorithms then calculate the optimal path from the trachea to the target lesion, considering the medical robot‘s physical constraints (diameter, bending radius). Challenges include handling respiratory motion simulation and efficiently planning paths for multiple nodules. Competitions like MICCAI’s ATM22 drive advancements in deep learning models for highly accurate and topologically sound airway segmentation.
- Image-Based Guidance: This dominant approach uses the medical robot‘s own endoscopic camera feed. Techniques include:
- 2D-3D Registration: Matching real-time endoscopic images with virtual views generated from the pre-operative 3D model. AI is increasingly used (e.g., Visual Similarity Networks, Kinematic Refinement Networks, spatial geometry-aware networks) to extract robust features, handle visual ambiguities (e.g., mucus, bubbles, repetitive anatomy), and estimate the scope’s position and orientation (pose) more accurately and efficiently, reducing reliance on brittle traditional similarity metrics.
- 3D-3D Registration & Visual Odometry: Estimating depth from the 2D video stream (using monocular depth estimation networks) or tracking the scope’s movement frame-by-frame (visual odometry/SLAM – Simultaneous Localization and Mapping) to build a local 3D map and register it to the global model. While potentially more accurate, computational complexity and speed remain challenges for real-time application. Dedicated datasets are being created to train and benchmark these algorithms.
- Sensor-Based Guidance: Complementing or replacing vision:
- Electromagnetic (EM) Tracking: A small EM sensor at the scope tip is tracked within a generated magnetic field (e.g., Monarch, superDimension). While relatively immune to visual obstructions, it can be distorted by metallic objects in the environment. Fusion with optical trackers or image data helps mitigate drift and improve accuracy.
- Fiber Bragg Grating (FBG) Shape Sensing: Optical fibers with embedded gratings run along the instrument. Changes in light wavelength reveal strain, allowing the entire shape of the scope to be reconstructed in real-time (e.g., Ion). This provides continuous shape information but can suffer from cumulative errors over long paths. Sensor fusion (EM + FBG + vision) is a key research focus for robust, drift-resistant navigation essential for reliable medical robot operation.
- Advanced Robot Control Systems: Translating human or AI intent into precise robotic motion within the delicate lung environment requires sophisticated control architectures:
- Master-Slave Control: The dominant paradigm. The surgeon operates a master console (e.g., joysticks, haptic interfaces, even touchless gesture control with AR overlays), and their movements are scaled and translated into motion for the slave medical robot inside the patient. Research focuses on improving fidelity, reducing latency, and incorporating force feedback to prevent tissue damage. Algorithms like IALO-BP-PID aim to enhance position tracking performance.
- Shared Control: This emerging paradigm combines human expertise with machine intelligence. AI algorithms analyze the surgeon’s inputs, the medical robot‘s state, and sensor data (vision, position, force) to predict intent, provide guidance (e.g., virtual fixtures preventing dangerous movements), or even semi-autonomously execute sub-tasks (e.g., maintaining position at a branch point, retracting a needle). Systems like the “AI Co-Pilot” developed by Lu Jiabao, Wang Yue, and Xiong Rong’s team learn from expert demonstrations to assist less experienced operators, enhancing safety and reducing errors. This collaborative approach leverages the strengths of both human and machine.
- Autonomous Control: The frontier. Research explores enabling the medical robot to perform specific complex tasks autonomously under high-level supervision. Examples include Kuntz’s work on autonomous steerable needle navigation through tissue for biopsy sampling and Li Jianmin’s team’s work on automated lumen center detection for bronchoscope insertion. While full autonomy for complex procedures like navigating all bronchial branches remains distant, task autonomy for specific steps (e.g., holding position, driving a straight path, precise needle insertion) is actively being developed, aiming to reduce surgeon cognitive load and procedural time.
Industry Momentum: From Lab Bench to Bedside
The bronchoscopic medical robot is rapidly transitioning from research prototypes to commercially available medical devices. The landscape features established players and ambitious newcomers:
- Navigation Systems: Companies like Medtronic (superDimension), Veran Medical (LungPoint/SPiN), and LungCare (China) offer EM or VBN navigation systems that enhance traditional bronchoscopy, providing real-time localization guidance.
- Integrated Robotic Platforms:
- Intuitive Surgical (Ion): Features a 3.5mm outer diameter scope with a 2.0mm tool channel, utilizing proprietary shape-sensing technology (likely FBG-based) for navigation, often combined with intra-procedural C-arm confirmation.
- Johnson & Johnson/Auris Health (Monarch): Utilizes a 4.2mm scope within a 6mm outer sheath (2.1mm tool channel), employing articulated “snake-drive” mechanics for navigation, primarily guided by EM tracking.
- Chinese Innovators: Companies like LungHealth (Edge Medical), MicroPort, Broncus, and others are making significant strides. Their platforms integrate flexible endoscopes, electromagnetic or virtual navigation, multi-degree-of-freedom robotic arms, and sophisticated software for 3D reconstruction, path planning, and visualization, aiming to provide cost-effective and advanced solutions. Figure 10 in the source material details comparative specs (end effector size, tool channel, navigation mode, certifications).
This burgeoning industry signifies strong confidence in the clinical and commercial potential of bronchoscopic medical robots. Regulatory approvals (FDA, NMPA) are accelerating, paving the way for broader clinical adoption.
Future Trajectory and Persistent Challenges
The potential of bronchoscopic medical robots is immense, but significant hurdles remain before their full capabilities are realized:
- Enhanced Dexterity and Miniaturization: Reaching the tiniest bronchioles demands even smaller, more flexible robotic instruments capable of large-angle bending without sacrificing stability or tool channel capacity. Developing novel biocompatible materials and ingenious miniaturized actuation mechanisms is crucial. Sensor miniaturization (EM, FBG, vision, force) is equally critical for deep lung navigation.
- Intelligent Multi-Modal Perception & Navigation: Future systems need seamless integration of diverse real-time data streams: endoscopic video, EM/FBG position, pre-operative CT/MRI, intra-operative ultrasound/CBCT, respiratory motion tracking, and force sensing. AI will be central to fusing this data, creating dynamic “living” 3D maps of the lungs during the procedure, compensating for breathing and organ shift in real-time, and enabling truly robust, drift-free navigation – especially vital for targeting moving lesions. Detailed anatomical labeling of bronchial branches within models will further enhance navigation.
- The Autonomy Conundrum: While shared control offers immediate benefits, achieving higher levels of autonomy for complex decision-making (e.g., autonomously selecting the correct branch at every bifurcation based on the planned path and real-time perception) is a major research goal. This requires breakthroughs in AI reasoning, real-time scene understanding under uncertainty, and fail-safe mechanisms. Ensuring absolute safety in autonomous or semi-autonomous modes is non-negotiable. The vision is a medical robot capable of autonomously navigating to a target, performing a biopsy, and potentially even initiating localized therapy under surgeon oversight.
- Integrated Diagnosis and Therapy: The future lies not just in diagnosis but in seamless therapy. Platforms are evolving towards combining precise lesion navigation, real-time biopsy with rapid on-site evaluation (ROSE), and immediate minimally invasive treatment (e.g., ablation, marker placement, local drug delivery) in a single, integrated robotic procedure. The medical robot becomes a comprehensive interventional platform.
- AI as the Co-Pilot’s Brain: AI integration will deepen beyond navigation assistance. Expect AI for automated lesion detection and characterization on endoscopic/virtual views, predictive analytics for optimal biopsy site selection based on imaging and patient data, personalized treatment planning, procedural outcome prediction, and enhanced surgeon training through simulation and performance analytics. AI will be the intelligent core augmenting the medical robot‘s capabilities.
- Proving Real-World Efficacy: Despite promising early results, large-scale, prospective, randomized controlled trials (RCTs) comparing robotic bronchoscopy head-to-head with established methods across diverse patient populations and healthcare settings are essential to definitively establish its clinical and cost-effectiveness. Long-term data on patient outcomes is also needed.
Conclusion: Precision Takes Flight
The development of bronchoscopic medical robots represents a paradigm shift in pulmonary medicine. By conquering the anatomical complexities that have hindered traditional approaches, these systems offer unprecedented access to the lung periphery, enabling earlier, more accurate diagnosis of deadly lung cancers and opening doors to novel minimally invasive therapies. The convergence of advanced robotics, sophisticated sensing, intelligent navigation algorithms, and artificial intelligence is propelling this field forward at a remarkable pace.
While challenges in miniaturization, robust autonomy, multi-modal perception, and comprehensive clinical validation persist, the trajectory is clear. The bronchoscopic medical robot is rapidly evolving from an assistive tool into an intelligent, integrated platform for diagnosis and intervention. As technology matures and clinical evidence mounts, these systems hold the profound promise of transforming lung cancer from a late-stage killer into a disease routinely detected and treated in its earliest, most curable stages, fundamentally improving patient survival and quality of life. The era of robotic precision within the human airways has decisively begun.
